Topics
Definition
Classification
Mechanism of lubrication
Properties
Solid lubricants
LUBRICANTS
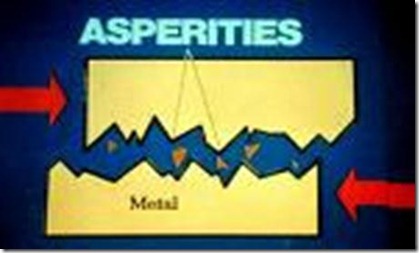
On viewing under an electron microscope, the surface of even a mirror polished metallic surface exhibits irregularity. The peaks are called asperities and the valleys are called pits
Scanning Electron Microscopic Picture of Mirror Polished Platinum Surface
Asperities and Valleys on two sliding surfaces
Types of Friction:
There are two types of friction (Static and Dynamic)
• Sliding Friction: Two flat Surfaces in motion
• Rolling Friction: At least one moving surface is spherical
Frictional Heat and Welded Joints
Ar= Real area of contact, junction, high stress, plastic deformation, adhesive bonding
B = Area filled with oxides, lubricant or air
Micro weld is the area where invisible welded joints are formed
N = Normal force
F = Frictional force
Definition
Substances introduced between moving surfaces in order to reduce friction and thus prevent welded joints and seizure, are called Lubricants
The process of prevention of metal to metal contact by means of an intervening layer of fluid or fluid like material is termed as Lubrication
Functions of Lubricants
It acts as a thermal barrier and reduces friction and wear and prevents welded joints
Avoids seizure of moving surfaces
Acts as coolant
Acts as a seal and prevents entry of dust, moisture, & dirt between moving parts
Some lubricants acts as corrosion inhibitors thus reduce operational cost
Classification:
Three Different types of classifications are done
Based on occurrence / Source
Based on lubricant Physical State
Based on Lubrication process
Liquid lubricant examples:
1. Animal oils - Lard oil, Tallow oil, Whale oil
2. Vegetable oil - Castor oil, Palm oil, Coconut oil
3. Mineral based - Petroleum fractions
4. Emulsions
O/ W Cutting liquids
W / O Cooling liquids
5. Synthetic oils – blended mineral oils
Thick Film / Fluid film / Hydrodynamic Lubrication
In this type a continuous fluid film exists between the sliding surfaces
Employed when the sliding velocity is moderate and load is low
Used for machineries operated continuously
In fluid film lubrication, the fluid immediately adjacent to each surface travels at the same speed and direction of each surface.
Film thickness is ≥1000º A
Factors which affect the thickness of hydrodynamic fluid film include lubricant viscosity, rotation speed or RPM, oil supply pressure, and component loading.
An increase in speed or viscosity increases oil film thickness.
An increase in load decreases oil film thickness.
Coefficient of friction is 0.001 to 0.03 cm/sec
Blended Mineral oils are used to introduce a thick film between sliding surfaces
Finds application in watches, clocks and scientific instrument (stop watches)
Hydrodynamic lubrication often occurs in components such as cylinders, gears, and plain bearings
Boundary Lubrication / Thin film
Boundary lubrication often occurs during the startup and shutdown of equipment, or when loading becomes excessive.
Low speed , high load & non continuous operation
Thickness is 20 – 30 A
Coefficient of friction is 0.05 – 0.15 cm /sec
Film formation by either Physisorption or chemisorption
Oiliness should be high
Fatty acids and their soaps are used as additives
Used in automobiles and heavy machinery
Certain types of gear sets that need to withstand sliding pressures and shock loading, such as hypoid gears use boundary lubricants
| |

a and b – Thick film lubrication
c and d - Thin film lubrication
Extreme Pressure Lubricants
Extreme pressure lubricants interact with metal surfaces in a chemical process, at molecular level, to create a protective compound which increases the thermal stability of metal surfaces.
This is not a film or coating over the metal. It is a permanent modification of the metal surface
Molecules in the extreme pressure lubricants are polarized and actually bond with the metal surface
It is applicable in High load and high speed condition
Metal surface should be active (Fe, Cu etc.,)
Lubricants containing Chlorinated ester/ Sulphurised oils / Tricresyl phosphate are used
Surface compounds is formed due to chemisorption (ex.sulphurised oil containing lubricants reacts with Iron and forms Iron sulphide whose thermal stability is better than mere iron)
The thickness of the surface compound is a few molecule layer
Co. efficient of friction 0.1 – 0.4 cm /sec
It finds application in Air crafts and space crafts
Unique character of this type of lubricants is its replenish ability / easy of replacement
Extreme pressure lubricant
Important Properties
1. Viscosity and Viscosity Index
Viscosity is defined as Resistance to flow
Viscosity Index is the measure of resistance to change in viscosity
Viscosity Index (V.I) is value representing the degree for which the oil viscosity changes with temperature.
If change in viscosity ( ie decrease in viscosity with increase in temperature) is small , the oil is said to have a high viscosity index.
Low viscosity does not mean Low VI (Ex. Viscosity of water is less but VI of water is high)
Viscosity Index is measured using Red wood (Common Wealth Countries)
Viscosity Index is measured using Say bolt viscometer (USA)
Viscosity Index is measured using Engler viscometer (European countries)
Naphthalenic base Gulf oils have Low viscosity index (VI =0 , L series oils)
Paraffinic base Pennsylvanian oils have high viscosity index (VI =100, H series oils)
The viscosity of test oil is measured at 100o F and 210 o F
An oil possessing the same viscosity as that of test oil at 210 o F
is chosen from both L and H series
Their corresponding viscosities at 100 o F is noted from the series ( L and H)
If the viscosity of the test oil at 100 o F is considered as U then its VI can be calculated using the formula
V.I. = (L – U / L – H) x 100
Polymers and copolymers of olefins, methacrylates, dienes or alkylated styrenes are used as viscosity index improvers
When the oil gets heated up due to frictional heat, the viscosity of the oil decreases.
The solubility of a polymeric molecule increases with increase in temperature which results in the increase in viscosity
Hence at elevated temperatures, the decrease in the viscosity of the oil will be balanced by the solubility of the polymer in oil. Thus polymers act as good VI improvers
Good motor oil has a high V.I.
2. Tackiness / oiliness / lubricity
Oiliness is defined as the ability of the oil to stick firmly to a solid surface
Oiliness should be high for a good lubricant
Can be improved using additives – long chain fatty acids and soaps are generally used as tackiness improvers
Very essential in Boundary lubrication as tackiness improves replenish ability of the surface film
3. Cloud and Pour Points
Cloud point is defined as the minimum temperature at which oil becomes hazy
Pour point is defined as the minimum temperature at which oil stops pouring( or ceases to flow)
When oil is cooled , wax starts crystallizing , and the oil becomes hazy
Tiny wax crystals agglomerate and form bigger crystal lattice, at this stage the oil solidifies completely and does not pour out on tilting.
Both cloud and Pour points should be very less than the operating temperature
Cloud and pour points can be reduced by adding depressants
Polymeric additives such as poly amino alcohols are added to decrease cloud and pour points
Other pour point depressants include alkylated naphthalene’s and phoenolic polymers, poly methacrylates, maleata/fumarate and copolymer esters
The additives lower the pour point either by forming a thin coating over wax and prevent it from agglomerisation or co precipitate along with wax , thus reducing the freezing point
(For diagram, description and working of cloud and pour point apparatus refer Text book - 194 OR class notes)
4. Flash and fire Points
Flash point is defined as the lowest temperature at which the oil gives off just enough vapours which gives out a flash when an open (tiny ) flame is brought near the vapours
Fire point is the lowest temperature at which the oil gives off enough vapours which burns continuously at least for 5 seconds
Flash and Fire points should be high for a good lubricant
Flash and fire point additives are used to increase the performance of a lubricant
CTFE is added to increase flash and fire points as it increases the vaporization point of lubricants
Usually there is a difference of 5 – 40o C between both for a good lubricant
Blended oils
Practically no single lubricant will be able to satisfy all the properties of a good lubricant. Hence it is essential to add various additives to achieve the required performance.
The process of adding different substances to impart a special character to the base oil is called blending and the resulting oil is called blended oil.
The following table gives the various additives and their function in synthetic oils / blended oils
| Name of the additive | Substance added to lubricating oils | Function |
| Oiliness | Fatty acids like stearic acid, palmitic acid, oleic acid Vegetable oils | Increases lubricity Prevent rupture of film |
| Extreme Pressure additives | Organic chlorine compounds / organic sulphur compounds / phosphorous compounds | Form surface compounds which has better thermal stability & wear resistance |
| Viscosity Index improvers | n-hexanol, polyalkyl benzene, polyisobutylene | Reduce the rate of change of viscosity with Temperature |
| Pour point depressants | Phenols, chlorinated hydrocarbons | Prevent the agglomerisation of wax which separates out from the lubricating oils |
| Thickeners | Polystyrene, polyesters | Increase the viscosity of lubricating oils |
| Antioxidants | Phenolic compds, aromatic amines | Prevent oxidation of oil. Prevent gum formation |
| Deposit inhibitors / detergents/ deflocculents | Salts of carboxylic acid / salts of phenols/ sulphonates | Reduce deposits in engines which block the passage of oil |
| Corrosion inhibitors | Organic compounds of Phosphorous / antimony | Get adsorbed on metal surface and protect the surface from attack by moisture |
Solid lubricants
Solid lubricants are deposited in the valleys to create a smoother surface.
They are designed to protect against metal contact by coming between two peaks at the moment of contact.
This results in the deformation of the peak, rather than formation of welded joints.
Even between highly loaded stationary surfaces the lamellar structure is able to prevent contact.
Large lubricant particles perform better on relative rough surfaces and at low speed, while finer particle perform better on smooth surface and higher speeds
(1st and 2nd are sliding surfaces and 3rd is solid lubricant filling the valley)
Layered Compounds like graphite , boron nitride ,molybdenum di sulphide , talc , teflon, mica, calcium fluoride , cerium fluoride, tungsten disulfide etc are as solid lubricants used
Graphite (organic substance) and molybdenum disulfide (MoS2 is inorganic substance) are the predominant materials used as solid lubricant due to their lamellar structure
The lamellas orient parallel to the surface in the direction of motion and shears over each other easily, resulting in a low friction.
Graphite
Graphite is a form of carbon which exists as a stack of 'sheets' of carbon atoms, each sheet having a hexagonal arrangement of atoms
Three valencies are satisfied by covalent bonds and the fourth is weak Vander
Waals Force
Both crystalline and amorphous form of graphite find application as lubricant, however amorphous graphite does not possess high shear strength hence used only when load is low.
Both natural and synthetic graphite can be used as lubricants
Graphite is soapy to touch and non inflammable
The C-C bond length is 1.42A and the distance between two hexagonal planes is
3.4A
It is stable up to 375oC (in presence of air)
Water vapor is a necessary component for graphite lubrication.
The adsorption of water reduces the bonding energy between the hexagonal planes of the graphite
Because water vapor is a requirement for lubrication, graphite is not effective in vacuum
Graphite is usually mixed with oil / water/ greases
Graphite mixed with oil is called oil dag and graphite mixed with water is called aqua dag
It is used to lubricate air compressors , railway track joints , food stuff industries , IC engines etc.,
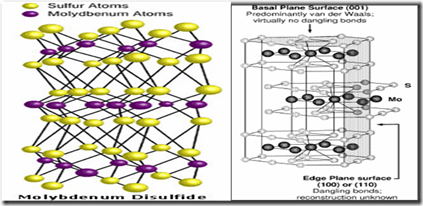
Molybdenum di Sulphide
Just like graphite, MoS2 also has a hexagonal crystal structure with the intrinsic property of easy shear
MoS2 lubrication performance often exceeds that of graphite and is effective in vacuum also
Mo layer is sandwiched between two sulphur layers
Like graphite it has weak van der Waals forces between the basal planes.
The bonds between the sulfur layers are weaker than the bonds between the molybdenum layers.
The Metallic bond length , Mo-Mo is 3.08A , the covalent bond length S-S is
3.15 A and the distance between two lamellas is 3.13A
It is stable up to 425oC in presence of air and up to 800oC in absence of air
It is chemically inert
It may be used in the form of powder/ mixed with oil or greases
Water is not essential for its lubricity hence can be used in vacuum too in fact its lubricity decreases in presence of water
It is used in IC engines.

















I agree with a lot of the points you made in this article. If you are looking for the Lubricants Dealers In Erode, then visit Mavani Synthetic Lubricants (P) Ltd. I love your content, they are very nice and very useful to us and this text is worth everyone’s attention.
ReplyDeleteGood post. I certainly love this website.
ReplyDeleteContinue the good work!
mini goldendoodle for sale
mini bernedoodle puppies for sale
mini aussiedoodles for sale
ragdoll cat for sale
ragdoll cats for sale
teacup havanese puppies for sale
great dane puppies for sale
great dane puppy for sale
great dane puppies near me
Hi, I do believe this is a great website. I stumbledupon it ;) I am going to revisit yet
ReplyDeleteagain since I book-marked it. Money and freedom is the greatest way to
change, may you be rich and continue to help other people.
great dane puppy for sale
great dane puppies for sale near me
goldendoodle for sale
ragdoll kitten near me
ragdoll kittens for sale near me
bernedoodles for sale
aussiedoodle for sale
havanese puppies for sale near me
Royalcasino310
ReplyDelete